Aluminum, a versatile and abundant metal, is often associated with lightweight and durable products. Yet, its electrical conductivity propels aluminum into the realm of vital components in electrical applications. This opening passage invites readers to delve into the fascinating world of aluminum’s electrical properties, providing an enriching and engaging experience.
Electrical conductivity plays a paramount role in power generation, transmission, and consumption. Aluminum, boasting a unique atomic structure, has carved a niche for itself in this critical arena. Discover how aluminum stacks up against other metals and its pivotal contributions to various sectors, including power generation, manufacturing, and transportation.
Introduction to Aluminum
Aluminum is a silvery-white, lightweight metal with the chemical symbol Al and atomic number 13. It is the third most abundant element in the Earth’s crust, making up around 8% of its mass.Aluminum’s position in the periodic table is in group 13 and period 3.
Its atomic weight is approximately 26.98 g/mol, which is relatively low compared to other metals such as iron (55.85 g/mol) or copper (63.55 g/mol). This low atomic weight contributes to aluminum’s low density, which is roughly 2.7 g/cm3, making it a popular choice for applications requiring a lightweight yet strong material.
Fundamental Properties of Aluminum
Aluminum has a few noteworthy fundamental properties that make it an attractive metal for various applications:
1. Lightweight
Aluminum is one-third the density of steel, making it an ideal choice for industries requiring lightweight materials without compromising strength, such as the aerospace and automotive sectors.
2. High-strength-to-weight ratio
Aluminum can be alloyed with other elements, allowing manufacturers to tailor its strength, corrosion resistance, and fabrication properties. This versatility makes it an excellent option for manufacturing lightweight, durable products.
3. Thermal conductivity
Aluminum is an excellent thermal conductor, which makes it suitable for use in various heat transfer applications, such as cookware, car radiators, and HVAC systems.
4. Electrical conductivity
Aluminum has good electrical conductivity and cost-effectiveness for use in electrical transmission lines, busbars, and other applications with lower mechanical stresses.
5. Non-magnetic
Aluminum is non-sparking and non-magnetic, which means it does not emit sparks when struck or have magnetic attraction. This makes it suitable for use in explosive environments and in magnetic resonance imaging (MRI) equipment.
Atomic Characteristics in Comparison to Other Metals
Aluminum’s atomic number is 13, which falls between two other key metals: sodium (Na, 11) and silicon (Si, 14). Due to its positioning, aluminum provides a balance between reactive and non-reactive behavior. Sodium is highly reactive and cannot be found in its pure form in nature, while silicon is semiconductive and often used in alloys and electronics rather than pure form.Considering aluminum’s atomic weight of 26.98 g/mol, it is around half the weight of iron (55.85 g/mol) and copper (63.55 g/mol), pointing to its low density and higher malleability than these two metals.In conclusion, aluminum has several desirable properties that make it a popular choice for a wide range of applications, including its low density, high strength-to-weight ratio, electrical and thermal conductivity, non-magnetic behavior, and abundance in the Earth’s crust.
While it shares space with other key metals in the periodic table, aluminum’s position and characteristics place it in a class of its own.
Electrical Conductivity of Metals
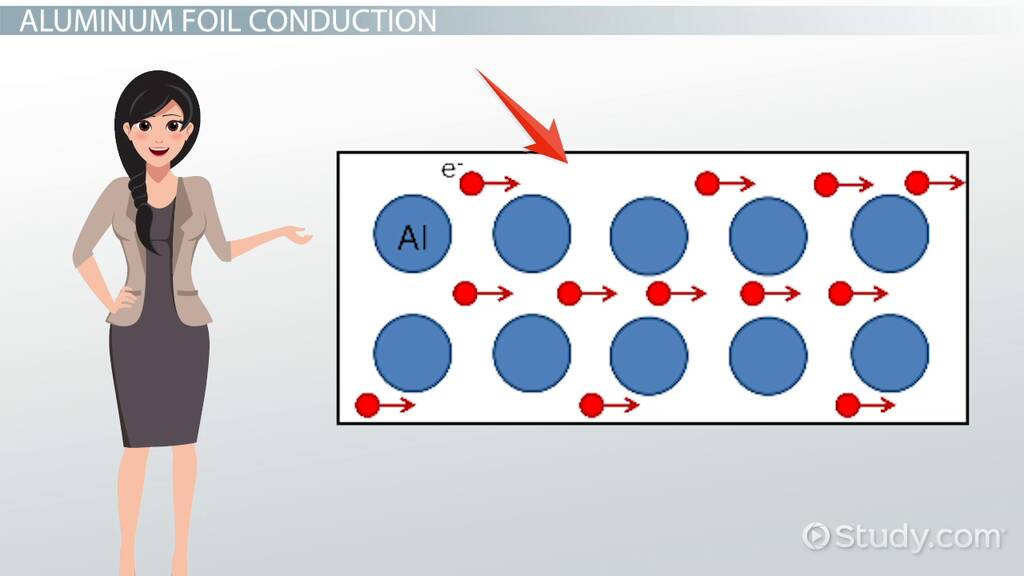
Electrical conductivity refers to a material’s ability to transmit electric current. It is typically measured in “Siemens per meter” (S/m). In this article, we’ll discuss how metals stack up as conductors.
Understanding Electrical Conductivity
Conductivity depends on a material’s electron density and the freedom with which these electrons can move. In metals, there is a “sea” of free electrons that can move easily under an electric field, allowing for electric current flow. Thus, metals are generally good conductors.
Conductivity Classification of Metals
Metals can be categorized as good, moderate, or poor conductors. Here’s a breakdown:
- Good conductors:Silver, copper, gold, and aluminum are prime examples.
- Moderate conductors:Steel, nickel, and lead fit this category.
- Poor conductors:Mercury, chromium, and tungsten are poor conductors among metals.
Factors Affecting Electrical Conductivity
Three key factors determine a metal’s conductivity:
- Number of valence electrons:Metals with more valence electrons (outermost electrons) usually have higher conductivity. For example, copper (Cu) has one more valence electron (1s 22s 22p 63s 23p 64s 1) than zinc (Zn) (1s 22s 22p 63s 23p 6), leading to higher conductivity in Cu.
- Lattice structure:A metal’s crystal structure can impact its conductivity. Face-centered cubic (FCC) structures generally exhibit higher conductivity than body-centered cubic (BCC) or hexagonal close-packed (HCP) structures.
- Temperature:Raising the temperature increases a metal’s resistance and lowers its conductivity. This phenomenon is due to the thermal motion of ions and the collisions between moving electrons and these ions.
Aluminum’s Place in Electrical Conductivity
Aluminum is a versatile metal with a wide range of applications due to its unique properties, including its electrical conductivity. This places aluminum among the most useful metals for various industrial and commercial purposes.
Classification of Aluminum in Terms of Electrical Conductivity
Aluminum is a good conductor of electricity, being in the group of elements calledType-I (or unalloyed) metals, which have a face-centered cubic (FCC) crystal structure. In general, Type-I metals have the highest electrical conductivity among all metals. Among these Type-I metals, aluminum has the second-highest conductivity, followed by copper, which stands out as the most electrically conductive element in this group.
Comparison of Aluminum Electrical Conductivity with Other Common Metals
When comparing aluminum’s electrical conductivity to other common metals, it is essential to consider the following table:| Metal | Electrical Conductivity (IACS %) ||—————–|————————————–|| Silver | 100 || Copper | 97.4 || Gold | 73.2 || Aluminum | 61.0 || Zinc | 28.7 || Lead | 7.8 |The above table illustrates aluminum’s electrical conductivity relatively to other metals.
It is measured in International Annealed Copper Standard (IACS) percentage. This value expresses the conductivity in relation to copper. For instance, aluminum has an IACS of 61.0%, implying it is 61.0% as conductive as annealed copper.
Applications of Aluminum in Electrical Applications due to Its Conductivity
Due to its electrical conductivity, aluminum is widely utilized in various industrial and commercial applications, which include:
- Transmission lines: Aluminum conductors have been extensively used for high-voltage power transmission lines. They provide benefits like lower costs, reduced weight, and better corrosion resistance compared to copper conductors with the same ampacity.
- Bus bars: Aluminum bus bars are a popular choice in power distribution systems to transport electrical currents among equipment, such as transformers, switchgears, or panelboards. Given their conductivity, they are also capable of minimizing energy losses.
- Motors: Aluminum is also present in various motor components, like conductors, rotors, and stators. Its usage in motors brings advantages like lower weight, reduced energy consumption, lower costs, and EMI (electromagnetic interference) reduction.
The Science Behind Aluminum’s Conductivity

Aluminum’s exceptional electrical conductivity can be attributed to its atomic structure and the free electron theory.
Atomic Structure of Aluminum
Aluminum has an atomic number of 13, making it a lightweight, silver-white metal. Its atomic structure has 13 protons and 14 neutrons in the nucleus, while the outer shell contains three electrons in the third energy level. This outermost energy level holds a maximum of eight electrons in a stable configuration.
Therefore, aluminum readily loses its three extra electrons during chemical reactions (
“Electron Configuration,” Encyclopædia Britannica
).
The Role of Free Electron Theory in Aluminum’s Conductivity
In the context of metals, the free electron theory explains the motion of valence electrons in the metal lattice. These valence electrons are only weakly bound to the atom and can move freely within the metal lattice. As a result, the electron mobility results in high electrical conductivity.
In aluminum, the presence of three free electrons in its atomic structure further contributes to its excellent conductive properties (
“Free Electron Model,” Encyclopædia Britannica
).
Valence Electrons in Aluminum vs. Other Metals
Notably, the number of valence electrons impacts the conductivity of a metal. For example, copper shares similar properties with aluminum and also has high electrical conductivity. Copper has one more valence electron in comparison with aluminum (29 protons vs. 13 protons).
Therefore, understanding the relationship between valence electrons and conductivity should be investigated more thoroughly. Factors including electron mobility, the atomic radius, and electronegativity might influence conductivity as well. These factors could provide more insights on how valence electrons contribute to high conductivity in various metals (
“Conductivity of Metals,” Chemistry LibreTexts
).
Resistance and Aluminum
Aluminum, a highly conductive metal, still exhibits a certain level of electrical resistance, which affects its ability to transmit electrical current. Resistance is the opposition encountered by an electric current as it flows through a conductor. This resistance is determined by the material’s inherent properties and the physical conditions of the conductor, namely length, cross-sectional area, and temperature.
In this section, we will explore the concept of electrical resistance and the factors that influence it in aluminum.
Understanding Electrical Resistance
Electrical resistance (R) is a measure of the opposition encountered by an electric current (I) as it flows through a conductor. This resistance depends on the material’s inherent properties, primarily its resistivity (ρ), and the conductor’s physical dimensions. The formula for resistance is:R = ρ × (L/A)where R is the resistance, ρ is the resistivity, L is the length, and A is the cross-sectional area of the conductor.Resistivity (ρ) is a measure of a material’s ability to resist the flow of electric current, quantifying the intrinsic opposition encountered by the current as it flows through the material.
It is expressed in ohm-meters (Ω⋅m). The electrical resistivity of materials is a primary factor in determining their suitability for various electrical applications.
Factors Affecting Electrical Resistance in Aluminum
Aluminum’s electrical resistance is primarily affected by its temperature and the physical dimensions of its conductors. We will delve into these factors in the following sections.
Temperature Dependence
Temperature has a significant impact on a material’s electrical resistance. In general, as the temperature of a conductor increases, its resistance also increases. This phenomenon occurs because increased thermal agitation of the conductor’s atoms impedes the movement of electrons, thereby increasing the resistance.Aluminum, a common conductor, is no exception.
Its resistivity increases with rising temperatures. The temperature coefficient (α) of resistance for aluminum denotes this relationship:ρ (T) = ρ (T0) × (1 + α × (T
T0))
where ρ(T) is the resistivity at temperature T, ρ(T0) is the resistivity at a reference temperature T0 (usually 20°C or 293.15K), and α is the temperature coefficient of resistance (approximately 0.0039°C−1 or 3.9 × 10−3 (K−1)).
Dimensional Dependence
The dimensions of an aluminum conductor significantly impact its resistance. Aluminum conductor manufacturers must account for this dependence in order to optimize their products’ electrical performance. The following factors contribute to dimensional dependence:
- Length:
The resistance of an aluminum conductor is directly proportional to its length (L). Increasing the conductor’s length, without changing its cross-sectional area (A) or temperature, will result in a higher resistance as the current must traverse a greater distance.
- Cross-sectional Area:
The resistance of an aluminum conductor is inversely proportional to its cross-sectional area (A). Enlarging the conductor’s cross-sectional area, while keeping its length and temperature constant, will lower its resistance since the increased area allows for a higher current density and a reduced concentration of collisions between electrons and atoms.
Comparing Aluminum’s Resistance with Other Metals
Aluminum generally exhibits lower electrical resistance than copper and higher resistance than silver. Copper and silver are traditionally valued for their lower resistance in electrical applications, but aluminum remains a cost-effective and practical alternative.
The electrical resistivity of aluminum (2.65 × 10−8 Ω⋅m) is lower than that of copper (1.68 × 10−8 Ω⋅m) at 20°C, making it an attractive option for use in transmission lines and electrical components where cost and weight are critical factors.
Nevertheless,Aluminum’s resistance is significantly higher than that of silver (1.59 × 10−8 Ω⋅m). The discrepancy arises from the differences in the materials’ inherent properties and crystal structures.
Overall, aluminum’s cost-effectiveness, corrosion resistance, and formability make it a popular choice for a wide range of electrical applications, despite its relatively higher resistance compared to other metals such as silver and copper.
Applications of Aluminum in Electrical Conductivity
Aluminum’s electrical conductivity has led to its widespread use in various industries, particularly in power transmission and electrical wiring. Its advantages over other metals, such as copper and steel, have made it a go-to material in many applications. This article will discuss some of the real-life examples of aluminum’s use in power lines, electrical wiring, and transformers.
Additionally, we will look at the benefits of using aluminum in electrical applications over other metals and the future growth and potential of aluminum in electrical conductivity applications.
Aluminum in Power Lines
Aluminum is commonly used in power lines due to its low density and resistance. Its lightweight property makes it easier and cheaper to install and maintain power lines than copper or steel, which are heavier and more cumbersome. Additionally, aluminum’s resistance to corrosion makes it an ideal choice for power lines exposed to harsh weather conditions.
Aluminum in Electrical Wiring
In addition to power lines, aluminum is also used in electrical wiring due to its high conductivity and cost-effectiveness. Although copper has higher conductivity than aluminum, aluminum is a more cost-effective option, especially in large-scale construction projects. Aluminum wiring is also flexible, making it easy to install and bend around corners, which is essential in construction sites.
Aluminum in Transformers
Aluminum is used in transformers, which are essential components in power transmission and distribution systems. Transformers need to be lightweight, compact, and efficient, making aluminum an ideal material. Aluminum’s high electrical conductivity and resistance to corrosion make it an excellent choice for transformers, especially in coastal areas where corrosion is a significant concern.
Advantages of Aluminum over Other Metals
Aluminum has several advantages over other metals, making it an ideal choice for electrical conductivity applications. Some of these advantages include:
- Low density: Aluminum is lightweight, making it easier and cheaper to install and maintain than heavier metals such as copper and steel.
- High conductivity: Despite its lower density, aluminum has high electrical conductivity, making it an excellent alternative to copper.
- Corrosion resistance: Aluminum is resistant to corrosion, which makes it ideal for power lines and other electrical applications exposed to harsh weather conditions.
- Cost-effective: Aluminum is more cost-effective than copper, making it an attractive option for large-scale construction projects.
Growth and Future Potential of Aluminum in Electrical Conductivity Applications
The use of aluminum in electrical conductivity applications is expected to grow due to its advantages over other metals. The increasing demand for renewable energy sources, such as wind and solar power, is driving the growth of aluminum in power transmission and distribution systems.
Additionally, the growing demand for cost-effective and energy-efficient electrical wiring in construction projects is expected to increase the use of aluminum in electrical wiring.
Moreover, advancements in technology are expected to increase the efficiency of aluminum in electrical conductivity applications. Innovations such as aluminum alloys with higher conductivity and strength than pure aluminum are expected to increase the use of aluminum in electrical conductivity applications.
Last Point
Aluminum’s extraordinary electrical conductivity, coupled with its distinct advantages over other metals, makes it an ideal candidate for electrical applications. The versatile metal’s future potential in conducting electricity remains promising as research and development continue to uncover novel applications and improvements.
Aluminum’s unique properties provide a captivating subject as the world continues to advance and innovate in the realm of electrical conductivity.